
Strategy Development for Accelerated Marketing Authorization and Early Market Access
The Rarer the Disease, the Greater the Challenge: Early market access strategies for transformative medicines in rare diseases are uncharted terrain, requiring highly specific expertise and experience.

Driven by the advent of precision medicine and empowered patients, the current healthcare landscape stresses the “need for speed” in biomedical innovation: more rapid development, accelerated and conditional approval, and – crucially – timely patient access to medical innovations. Here “the science has overtaken the system”: innovations are coming to market with ever fewer data (e.g. Phase II POC) and uncertainties whether the anticipated clinical benefit will be verified in real-world practice and possibly undiscovered risks pose challenges to payers and HTA bodies. This can result in delays or restrictions in access to potentially transformative treatments for patients with debilitating and life-threatening conditions, whose prime focus it is to improve their quality of life.
Developers with orphan and advanced therapeutics in their pipeline, which may be eligible for accelerated approval, should therefore consider implementing effective early access strategies, which are crucial to informing, enhancing and supporting strategic thinking on
- Innovative, accelerated pathways to bring treatments to patients in the most timely manner
- Generating meaningful patient-relevant outcomes through early and sustained patient engagement
- Early value demonstration specifically addressing the challenges of early market access with fewer data
- Differentiated value-based offerings with the greatest net benefit for successful pricing and reimbursement
- Maximising product value from earlier launch to loss of exclusivity with a life-cycle full-spectrum evidence generation strategy
- Addressing burgeoning R&D expenses and competitive situations with a "fast to market" approach
Early access strategy is developed through research, innovative thinking, and interactive discussion on the following key elements:
Unmet Medical Need
- Both disease and treatment related characteristics are crucial in defining the value of a novel treatment. Therefore, a comprehensive documentation of the burden of disease and the unmet patient needs provides the basis for all stakeholder communication.
- Unmet need is understood and described from a solid foundation of knowledge in epidemiology (rarity), natural disease history, diagnosis, standards of care, real-world effectiveness, patient-relevant outcomes, pivotal research and insights on patient preferences.
- In the context of early access, the potential urgency and immediacy of the unmet patient need is a key factor.
Promise
- A medicine’s eligibility for early access is based on its intention to treat a serious condition and a credible promise of significant improvements in clinical benefit and patient-relevant outcome(s) over existing treatment.
- Very early in development, evidence of the potential to address unmet medical need may be demonstrated in a nonclinical model or pharmacologic data, for example. Later, preliminary clinical data should indicate the drug’s potential.
Orphan Feasibility
- Many products fulfilling the criteria for orphan designation will also qualify for early access. Therefore, the feasibility of orphan drug designation should be evaluated as part of any early access strategy, and vice-versa.
- Coordinated early access and orphan designation strategies are particularly crucial if a medicine has the potential for significant benefit in both rare and non-rare conditions or multiple orphan subsets.
Value
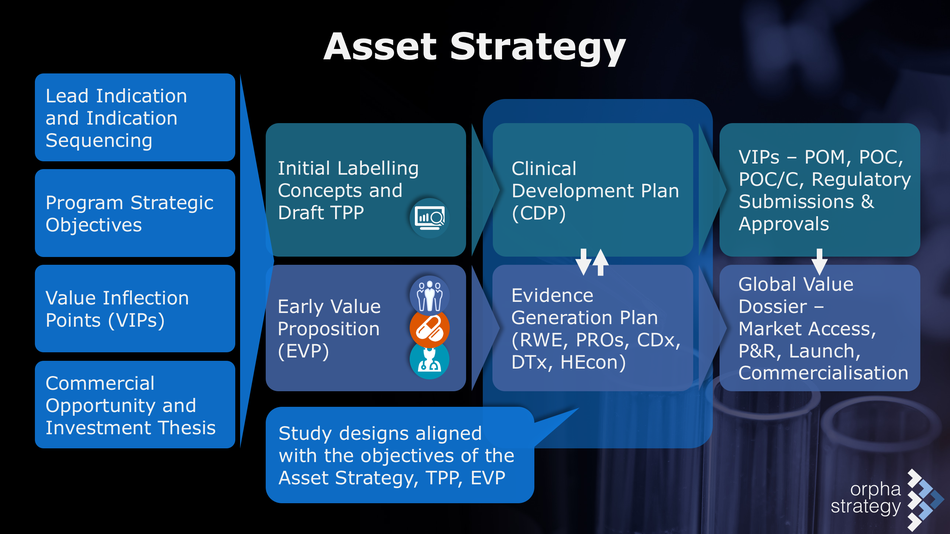
- Given that it does not seem rational to commit resources to accelerated marketing authorisation if timely market access cannot be achieved, planning for early value demonstration is arguably the most essential component of early access and should include:
-
- Documenting comprehensively and compellingly the burden of disease and the urgency of unmet patient needs
- Contextualising the promise and degree of innovation of the novel medication in addressing some of these unmet needs as compared to existing treatments in an early value proposition
- Mapping out the care pathway, the current standards of care, and the patient’s journey
- Identifying those patients with the greatest need and which will benefit the most to demonstrate the maximal net benefit of the novel treatment over standard of care
- Developing a value evidence generation strategy in coordination with the clinical development plan
- Seeking early HTA advice, exploring the options of parallel EMA/HTA scientific advice, Adaptive Pathways, PRIME, EUnetHTA JA 3
- Conceptualising study designs and endpoints that are relevant and actionable for both regulatory and HTA decision-making.
- Describing the benefits of the immediate availability of the medication to patients, physicians and health care providers, the healthcare system, public health, and society at large
- “Pressure testing” the early value proposition and early global value dossier (eGVD) against recognised value assessment and HTA guidelines, e.g. ORPH-VAL and/or MoCA-OMP for orphan medicinal products
- Proactively discussing remaining uncertainties in clinical benefits, risks and value and how these will be addressed with continued evidence generation post-authorisation
Real-World Evidence
- Stakeholders are increasingly focussed on expanding the toolbox for evidence generation, with pragmatic and real-world studies complementing RCTs
- Observational studies should ask the right questions, be underpinned by sound methodology, adhere to current guidelines, and provide a design and endpoint(s) that are relevant and actionable for regulatory and HTA decision-making
- Real-world evidence should should be integrated and pre-planned early on in a life-cycle approach throughout the development process, particularly including post-authorisation
- Innovative adaptive study designs and development pathways emphasising the application of real-world evidence should be considered in the development of an early access strategy. Further information: Real-World Research.
Patient Centricity and Engagement
- Meaningful, patient-relevant outcomes are crucial to documenting unmet need and in demonstrating the net benefits of innovative medications in addressing this need, as compared to current alternatives.
- The rising empowerment and influence of patients, caregivers and patient advocates in healthcare decision-making emphasises patient preferences in both benefit/risk and value judgements of therapeutic innovations.
- Early engagement and partnering with patients to gain their insights and input throughout the development continuum, for example with the establishment of a patient advisory board and by building relationships with international patient communities and organisations, is a cornerstone of early access strategy.
Compassionate Use (Expanded Access)
- Compassionate use aims to facilitate the early access to investigational medicinal products for seriously ill patients that cannot be treated satisfactorily or that cannot enrol in ongoing clinical trials.
- Many products fulfilling the criteria for early access support and orphan designation will also qualify for compassionate use (cohort or named patient programmes) in the individual EU member states (or FDAs expanded access in the US).
- Therefore, the feasibility of a compassionate use strategy, potentially incorporating real-world data collection, should be evaluated as part of a comprehensive early access strategy.
- The approximate time between EMA authorisation and final pricing and reimbursement approval and commercialisation in the EU5 markets is 15 months. A paid-for compassionate use program is therefore a potential option to address both unmet patient needs as well as generate early revenues, particularly for innovative small- and mid-sized companies invested in rare and ultra-rare diseases.
Uncertainties
- Early in development there will be a number of caveats, risks and unknowns that may impact strategy development and decision-making. It is recommendable to proactively identify and address the most crucial missing information in a gap analysis.
- Uncertainties in clinical and value outcomes are of great concern to stakeholders: for example, for patients and physicians the possibility of undiscovered adverse events, and for payers who wish to avoid paying high prices for medicines that are less effective in real-world practice than anticipated.
- It is therefore crucial to have an iterative evidence generation plan in place, which includes post-authorisation, and to communicate these firm commitments to address any remaining uncertainties to the stakeholders.
Regulatory Context
- The EMA’s Support for Early Access and the FDA’s Expedited Programs provide the regulatory framework for early access in Europe and the US. To these are added national programs for early patient access and compassionate use in the various EU member states (expanded access in the US). The programs are discussed in detail here: Early Access.

Collaborative Strategy Development
ORPHA Strategy Consulting wishes to develop long-term partnerships with clients, for an individual compound or at the portfolio level. Every potential early access strategy is different. Success thus originates through collaboration, by merging our specific expertise with that of the client. We pride ourselves on our responsiveness to clients’ needs and our approach will flexibly adapt to individual requirements.
This favours an iterative and highly interactive strategy development process :

Thank you for your interest. To start a strategic discussion on early access, please contact David Schwicker.
Executive Briefings
ORPHA Strategy regularly publishes Executive Briefings which focus on news and current trends in early access, adaptive development pathways, transformative medicines, orphan drugs and designations, rare diseases, and real-world patient-centered evidence. They are available for download here: ORPHA Executive Briefings
About Us
We provide creative and analytically rigorous advice on strategic problems and devise effective solutions to drive our clients' programmes forward. We do this through collaboration, by channelling and merging our expertise with that of our clients. Our approach has been honed in more than 25 years of consulting experience and will flexibly adapt to specific and unique challenges. Please contact us today to start a strategic discussion about early market access strategy.
ORPHA Strategy Consulting
Phase IV Programs LLC
David Schwicker, Principal
Centralbahnstrasse 7
4051 Basle, Switzerland
Tel: +43 676 362 9571
www.linkedin.com/in/orphastrategy
